|
|
 Plant Environmental Responses Laboratory
Plant Environmental Responses Laboratory
|
Plant growth and development are highly regulated by environmental light conditions. We are analyzing these photoregulatory processes in the moss Physcomitrella, the fern Adiantum, and the model plant Arabidopsis.
|
|
Asc Prof |
Takeshi Kanegae |
e-mail |
|
|
|
Photomorphogenesis
|
|
Unlike animals, plants cannot easily move from the place where they are grown, and so they have withstand changing environmental conditions in order to survive there. Plants have many abilities, however, to sense environmental changes particularly through light information. They can "see" the environment through photo-sensor pigments, and adapt to it by regulating their growth and development. This phenomenon is called photomorphogenesis. Plants have three classes of sensor pigments, phytochromes, cryptochromes and phototropins. We are analyzing photomorphogenic responses at the cell level from the light absorption by the sensor to the cytoskeletal control of cell morphogenesis.
|
|
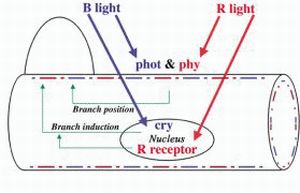
Intracellular localization of sensor pigments regulating branch photoinduction in the moss Physcomitrella patens
|
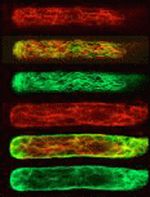
Actin filaments (red) and microtubules (green) in a protonemal cell of the fern Adiantum capillus-veneris
|
|
|
|
Chloroplast photomovement
|
|
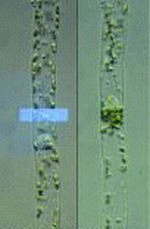
Chloroplast photorelocation in a protonemal cell of the fern Adiantum capillus-veneris
Interestingly, photomorphogenic sensors, phytochromes and phototropins, also regulate intracellular positioning of chloroplasts. Under weak or moderate light conditions, chloroplasts accumulate towards the illuminated area to maximize photosynthetic activity, while they escape from there under strong light conditions to avoid damage to the photosynthetic apparatus. We are analyzing the mechanisms of chloroplast photorelocation movements.
|
|
|
Recent Publications
|
|
- Kanegae T. (2015) Intramolecular co-action of two independent photosensory modules in the fern phytochrome 3. Plant Signal Behav (in press) DOI: 10.1080/15592324.2015.1086857
- Kanegae T, Kimura I. (2015) A phytochrome/phototropin chimeric photoreceptor of fern functions as a blue/far-red light-dependent photoreceptor for phototropism in Arabidopsis. Plant J. 83: 480-488.
- Sato Y, Kadota A. (2012) Fluorescence time-lapse imaging system equipped with a microbeam irradiator revealed a unique actin-based mechanism for chloroplast movement. Plant Morphology 24: 19-22.
- Yamashita H, Sato Y, Kanegae T, Kagawa T, Wada M, Kadota A.(2011) Chloroplast actin filaments organize meshwork on the photorelocated chloroplasts in the moss Physcomitrella patens. Planta. 233: 357-368
- Sugiyama Y, Kadota A. (2011) Photosynthesis-dependent but neochrome 1-independent light positioning of chloroplasts and nuclei in the fern Adiantum capillus-veneris. Plant Physiol 155: 1205-1213.
- Ichikawa S, Yamada N,Suetsugu N, Wada M, Kadota A. (2011) Red light, phot1 and JAC1 modulate phot2-dependent reorganization of chloroplast actin filaments and chloroplast avoidance movement. Plant Cell Physiol 52: 1422-1432.
- Yamada N, Suetsugu N, Wada M, Kadota A. (2011) Phototropin-dependent biased relocalization of cp-actin filaments can be induced even when chloroplast movement is inhibited. Plant Signal Behav 6: 1651-1653.
- Kadota A, Yamada N, Suetsugu N, Hirose M, Saito C, Shoda K, Ichikawa S, Kagawa T, Nakano A, Wada M. (2009) Short actin-based mechanism for light-directed chloroplast movement in Arabidopsis. Proc. Natl. Acad. Sci. USA 106: 13106-13111.
- Uenaka H, Kadota A. (2008) Phototropin-dependent weak and strong light responses in the determination of branch position in the moss Physcomitrella patens. Plant Cell Physiol. 49: 1907-1910.
- Uenaka H, Kadota A. (2007) Functional analyses of the Physcomitrella patens phytochromes in regulating chloroplast avoidance movement. Plant J. 51: 1050-1061.
- Kanegae T, Hayashida E, Kuramoto C, Wada M. (2006) A single chromoprotein with triple chromophores acts as both a phytochrome and a phototropin. Proc. Natl. Acad. Sci. USA 103: 17997-18001.
- Uenaka, H, Wada M, Kadota A. (2005) Four distinct photoreceptors contribute to light-induced side branch formation in the moss Physcomitrella patens. Planta 222: 623-631.
- Kawai-Toyooka H, Kuramoto C, Orui K, Motoyama K, Kikuchi K, Kanegae T, Wada M. (2004) DNA interference: a simple and efficient gene-silencing system for high-throughput functional analysis in the fern Adiantum. Plant Cell Physiol. 45: 1648-1657.
- Oikawa K, Kasahara M, Kiyosue T, Kagawa T, Suetsugu N, Takahashi F, Kanegae T, Niwa Y, Kadota A,Wada M. (2003) CHLOROPLAST UNUSUAL POSITIONING 1 is essential for proper chloroplast positioning. Plant Cell 15:1-12.
- Kawai H, Kanegae T, Christensen S, Kiyosue T, Sato Y, Imaizumi T, Kadota A, Wada M. (2003) Red light-induced responses in ferns mediated by an unconventional photoreceptor. Nature 421: 287-290.
- Sato Y, Wada M, Kadota A. (2003) Accumulation response of chloroplasts induced by mechanical stimulation in Bryophyte cells. Planta 216: 772-777.
- Imaizumi T, Kadota A, Hasebe M, Wada M. (2002) Cryptochrome light signals control development to suppress auxin sensitivity in the moss Physcomitrella patens. Plant Cell 14: 373-386.
- Sato Y, Wada M, Kadota A. (2001) External Ca2+ is essential for chloroplast movement induced by mechanical stimulation but not by light stimulation. Plant Physiol. 127: 497-504.
- Sato Y, Wada M, Kadota A. (2001) Choice of tracks, microtubules and/or actin filaments, for chloroplast photo-movement is differentially controlled by phytochrome and a blue light receptor. J. Cell Sci. 114: 269-279.
- Kadota A, Sato Y, Wada M. (2000) Intracellular chloroplast photorelocation in the moss Physcomitrella ptens is mediated by phytochrome as well as by a blue-light receptor. Planta 210: 932-937.
- Imaizumi T, Kanegae T, Wada M. (2000) Cryptochrome nucleocytoplasmic distribution and gene expression are regulated by light quality in the fern Adiantum capillus-veneris. Plant Cell 12: 81-96.
- Sato Y, Kadota A. (2006) Plastid movements in response to environmental signals. In: The Structure and Function of Plastids, Advances in Photosynthesis and Respiration Vol. 23 (ed. by Wise RR, Hoober K.) Springer
- Kanegae T, Wada M. (2006) Photomorphogenesis of ferns. In: Photomorphogenesis in Plants and Bacteria- Function and Signal Transduction Mechanisms. (3rd ed.) (ed. by Schafer E, Nagy F.)Springer, Dordrecht, pp. 515-536.
- Sato Y, Kadota A, Wada M. (2003) Chloroplast movement: Dissection of events downstream of photo- and mechano-perception. J. Plant Res. 116: 1-5.
|
|
|