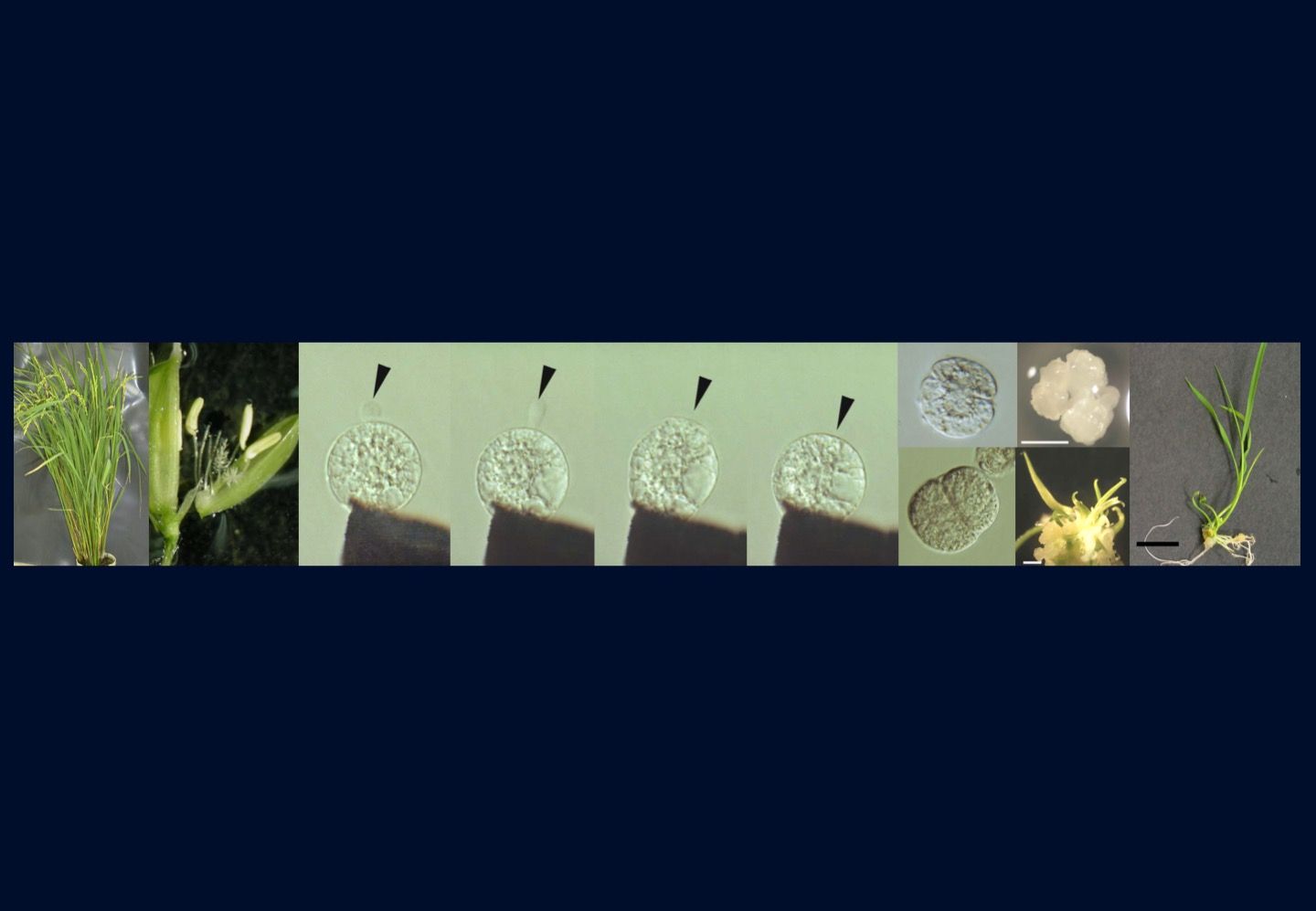
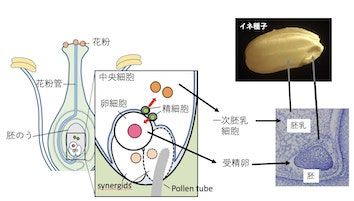
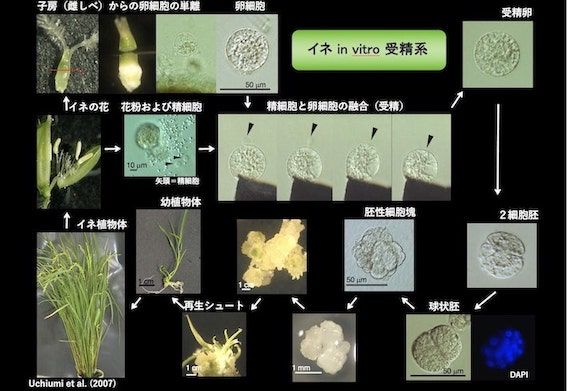
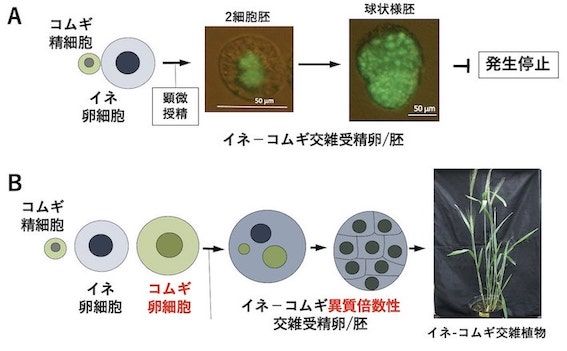
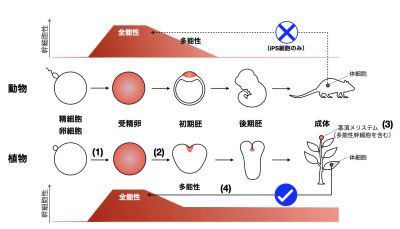
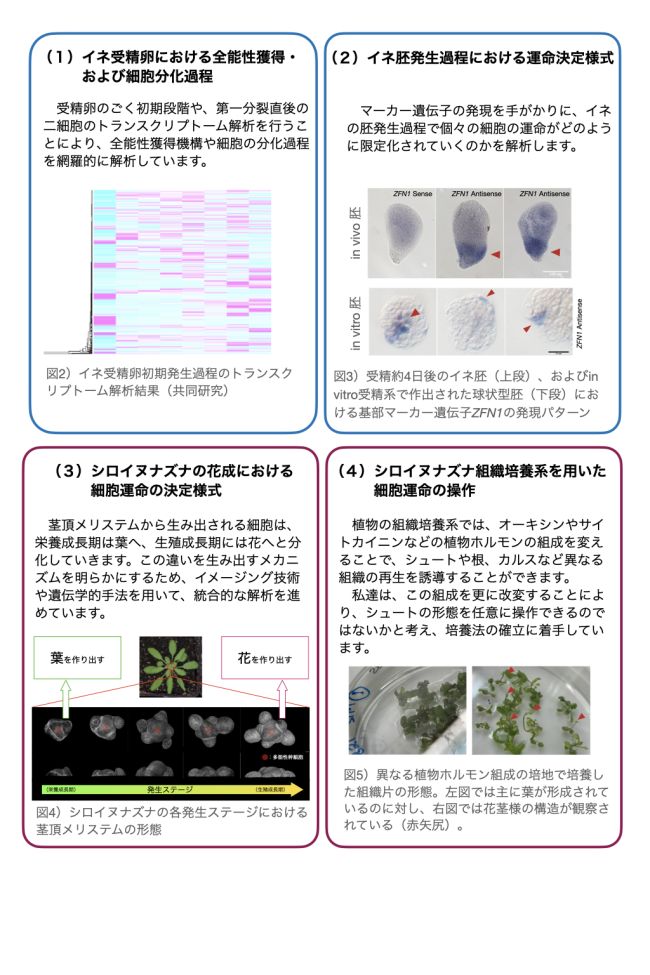
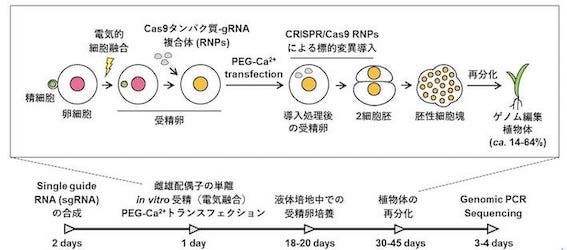
研究室メンバー および 研究業績
教授 岡本 龍史
助教 古川 聡子
助教 木下 温子
博士後期課程 渡辺 選子、Kasidit Rattanawong、Tety Maryenti、Hanifah Aini
博士前期課程 赤坂 大輔、澤本 陸、増尾心之介、渡辺 真史
卒業研究 鈴木綾佳、姫野翔
客員准教授 瀬尾光範
客員研究員 加藤 紀夫、内海 貴夫、大西由之佑
【原著論文】
Aini H., Sato Y., Uno K., Higashiyama T., *Okamoto T. (2021) Dynamics of mitochondrial distribution during development and asymmetric division of rice zygotes. Plant Reproduction, in press
Maryenti T., Ishii T., *Okamoto T. (2021) Development and regeneration of wheat–rice hybrid zygotes produced by in vitro fertilization system. New Phytologists. in press
Rattanawong K., Koiso N., Toda E., Kinoshita A., Tanaka M., Tsuji H., *Okamoto T. (2021) Regulatory functions of ROS dynamics via glutathione metabolism and glutathione peroxidase activity in developing rice zygote. Plant J. in press
Maryenti T., Kato N., Ichikawa M., *Okamoto T. (2021) In vitro fertilization system using wheat gametes by electric fusion. Methods Mol. Biol., in press.
Deushi R., Toda E., Koshimizu S., Yano K., *Okamoto T. (2021) Effect of paternal genome excess on the developmental and gene expression profiles of polyspermic zygotes in rice. Plants, 10:255.
*Toda E., *Okamoto T. (2020) Gene expression and genome editing systems by direct delivery of macromolecules into rice egg cells and zygotes. Bio Protocol 10: e3681.
*Toda E., *Okamoto T. (2020) A CRISPR/Cas9-based genome-editing using rice zygotes. Curr. Protocol Plant Biology, 5: e20111
Rahman MH, Toda E., *Okamoto T. (2020) In vitro production of zygotes by electro-fusion of rice gametes. Methods Mol. Biol., 2122: 257-267.
*Ohnishi Y., Kokubu I., Kinoshita T., Okamoto T. (2019) Sperm entry into the egg cell induces the progression of karyogamy in rice zygotes. Plant Cell Physiol., 60: 1656-1665.
*Toda E., Koiso N., Takebayashi A., Ichikawa M., Kiba T., Osakabe T., Osakabe Y., Sakakibara H., Kato N., *Okamoto T. (2019) An efficient DNA- and selectable marker-free genome editing system using zygotes in rice. Nature Plants 5: 363–368.
Rahman MH, Toda E., Kobayashi M., Kudo T., Takahara M., Iwami M, Watanabe Y., Sekimoto H., Yano K., *Okamoto T. (2019) Expression of genes from paternal alleles in rice zygotes and involvement of OsASGR-BBML1 in initiation of zygotic development. Plant Cell Physiol. 60: 725–737.
Maryenti T., Kato N., Ichikawa M., *Okamoto T. (2019) Establishment of an in vitro fertilization system in wheat (Triticum aestivum L.). Plant Cell Physiol. 60: 835-843.
Kim MY., Ono A., Scholten S, Kinoshita T., *Zilberman D., *Okamoto T., *Fischer R. (2019) DNA demethylation by ROS1a in rice vegetative cells promotes methylation in sperm. Proc. Natl. Acad. Sci. USA 116: 9652-9657.
Toda E., Ohnishi Y., *Okamoto T. (2018) An imbalanced parental genome ratio affects the development of rice zygotes. J. Exp. Bot. 69: 2609-2619.
Sukawa Y., *Okamoto T. (2018) Cell cycle in egg cell and its progression during zygotic development in rice. Plant Reprod. 31: 107-116.
Yamamoto T., Yoshida Y., Nakajima K., Tominaga M., Gyohda A., Suzuki H., Okamoto T., Nishimura T., Yokotani N., Minami E., Nishizawa T., Miyamoto K., Yamane H., Okada K., *Koshiba T. (2018) Antagonistic regulation of RSOsPR10 expression by jasmonate/ethylene and salicylate pathways is mediated by OsERF87 activator and OsWRKY76 Repressor, respectively, in rice roots. Plant Direct 2: e00049.
Koiso N., Toda E., Ichikawa M., Kato N., *Okamoto T. (2017) Development of gene expression system in egg cells and zygotes isolated from rice and maize. Plant Direct 1: e00010
*Bowman JL., *Kohchi T., *Yamato KT., 79 authors, Okamoto T., 27 authors, Schmutz J. (2017) Insights into land plant evolution garnered from the Marchantia polymorpha genome. Cell 171: 287–304.
*Ohnishi Y. and Okamoto T. (2017) Nuclear migration during karyogamy in rice zygotes is mediated by continuous convergence of actin meshwork toward the egg nucleus. J. Plant Res. 130:339-348.
*Okamoto T. (2017) Analysis of proteins enriched in rice gamete. Methods Mol. Biol. 1669: 251-263.
Park K., Kim Y., Vickers M., Park JS., Hyun Y., *Okamoto T., *Zilberman D., *Fischer R., *Feng X., *Choi Y., *Scholten S. (2016) DNA demethylation is initiated in the central cells of Arabidopsis and rice. PNAS, 113: 15138-15143.
Toda E., Ohnishi Y., *Okamoto T. (2016) Electro-fusion of gametes and subsequent culture of zygotes. Bio Protocol, 6: e2074
Toda E., *Okamoto T. (2016) Formation of triploid plants via possible polyspermy. Plant Signaling & Behavior 11: e1218107.
Toda E., Ohnishi Y., *Okamoto T. (2016) Development of polyspermic rice zygotes. Plant Physiol. 171: 206-214
Matsumura T., *Okamoto T. (2016) Isolation of gametes from Brachypodium distachyon. Plant Biotech. 33: 39-43
Suzuki H., Yokawa K., Nakano S., Yoshida Y., Fabrissin I., Okamoto T., Baluška F., *Koshiba T. (2016) Root cap-dependent gravitropic U-turn of maize root requires light-induced de novo auxin biosynthesis via YUC-pathway in root apex. J. Exp. Bot. 67: 4581-4591.
Takeuchi K., Hasegawa H., Gyohda A., Komatsu S., Okamoto T., Okada K., Terakawa T., *Koshiba T. (2016) Overexpression of RSOsPR10, a root-specific rice PR10 gene, confers tolerance against drought stress in rice and drought and salt stresses in bentgrass. Plant Cell, Tissue and Organ Culture (PCTOC) 127:35–46
Ohnishi Y., *Okamoto T. (2015) Karyogamy in rice zygotes: Actin filament-dependent migration of sperm nucleus, chromatin dynamics, and de novo gene expression. Plant Signaling & Behavior, 10: e989021
Ohnishi Y., *Okamoto T. (2015) Microscopic observation, three-dimensional reconstruction, and volume measurements of sperm nuclei. Bio Protocol, 5: e1437,
Ohnishi Y., Hoshino R., *Okamoto T. (2014) Dynamics of male and female chromatin during karyogamy in rice zygotes. Plant Physiol. 165: 1533–1543
Abiko M., Furuta K., Yamauchi Y., Fujita C., Taoka M., Isobe T., *Okamoto T. (2013) Identification of proteins enriched in rice egg or sperm cells by single-cell proteomics. PLoS ONE 8(7): e69578
Abiko M., Maeda H., Tamura K., Hara-Nishimura I., *Okamoto T. (2013) Gene expression profiles in rice gametes and zygotes: Identification of gamete-enriched genes and up- or down-regulated genes in zygotes after fertilization. J. Exp. Bot. 64: 1927–1940
*Okamoto T. (2011) In vitro fertilization with isolated rice gametes: production of zygotes and zygote and embryo culture. Methods Mol. Biol. 710: 17-27.
Ohnishi T., Takanashi H., Mogi M., Takahashi H., Kikuchi H., Yano K., Okamoto T., Fujita M., Kurata N., *Tsutsumi N. (2011) Distinct gene expression profiles in egg and synergid cells of rice as revealed by cell type-specific microarrays. Plant Physiol. 155: 881-891.
Takeuchi K., Gyohda A., Tominaga M., Kawakatsu M., Hatakeyama A., Ishii N., Shimaya K., Nishimura T., Riemann M., Nick P., Hashimoto M., Komano T., Endo A., Okamoto T., Jikumaru Y., Kamiya Y., Terakawa T., *Koshiba T. (2011) RSOsPR10 expression in response to environmental stresses is regulated antagonistically by jasmonate/ethylene and salicylic acid signaling pathways in rice roots. Plant Cell Physiol. 52: 1686–1696.
*Okamoto T. (2010) Gamete fusion site on the egg cell and autonomous establishment of cell polarity in the zygote. Plant Signaling & Behavior, 5: 1464-1467.
Nakajima K., Uchiumi T., *Okamoto T. (2010) Positional relationship between the gamete fusion site and the first division plane in the rice zygote. J. Exp. Bot. 61: 3101-3105.
Sato A., Toyooka K., *Okamoto T. (2010) Asymmetric cell division of rice zygotes located in embryo sac and produced by in vitro fertilization. Sex Plant Reprod. 23: 211–217.
*Uchiumi T., Okamoto T. (2010) Rice fruit development is associated with an increased IAA content in pollinated ovaries. Planta 232: 579-592.
Takanashi H., Ohnishi T., Mogi M., Okamoto T., Arimura S., *Tsutsumi N. (2010) Studies of mitochondrial morphology and DNA amount in the rice egg cell. Curr. Genet. 56:33-41.
Wang S., *Okamoto T. (2009) Involvement of polypyrimidine tract-binding protein (PTB) related proteins in pollen germination in Arabidopsis. Plant Cell Physiol. 50: 179-190.
*Kranz E., Hoshino Y., Okamoto T. (2008) In vitro fertilization with isolated higher plant gametes. Methods Mol. Biol. 427: 51-69.
Uchiumi T., Uemura I., *Okamoto T. (2007) Establishment of an in vitro fertilization system in rice (Oryza sativa L.) Planta 226:581-589.
Uchiumi T., Shinkawa T., Isobe T., *Okamoto T. (2007) Identification of the major protein components of rice egg cells. J Plant Res. 120: 575-579.
Uchiumi T., Komatsu S., Koshiba T., *Okamoto T. (2006) Isolation of gametes and central cells from Oryza sativa L. Sex Plant Reprod. 19: 37-45.
*Okamoto T., Scholten S., Lörz H., Kranz E. (2005) Identification of genes that are up- or down-regulated in the apical or basal cell of maize two-celled embryos and monitoring their expressions during zygote development by a cell manipulation- and PCR-based approach. Plant Cell Physiol. 46: 332-338.
*Okamoto,T., Higuchi K., Shinkawa T., Isobe T., Lörz H., Koshiba T., Kranz E. (2004) Identification of major proteins in maize egg cells . Plant Cell Physiol. 45: 1206-1212.
Okabe T., Sutoh K., Okamoto T., Minamikawa T., *Yamauchi D. (2004) Hormonal regulation of expression of the gene for pod storage protein in common bean plants. Plant Biotec. 21: 215-223.
*Okamoto T., Shimada T., Hara-Nishimura I., Nishimura M., Minamikawa T. (2003) C-terminal KDEL sequence of a vacuolar KDEL-tailed cysteine proteinase (SH-EP) is essential for vacuolar transport of SH-EP and formation of KDEL-vesicle (KV). Plant Physiol. 132: 1892-1900.
*Okamoto T., Toyooka K., Minamikawa T. (2001) Identification of a membrane-associated cysteine protease (MCP) with possible dual roles in the endoplasmic reticulum and protein storage vacuole. J. Biol. Chem. 276: 742-751.
Minamikawa T., Toyooka K., *Okamoto T., Hara-Nishimura I., Nishimura M. (2001) Degradation of ribulose 1,5-bisphosphate carboxylase/oxygenase by vacuolar enzymes of senescing French bean leaves: Immunocytochemical and ultrastructural observations. Protoplasma. 218: 144-153.
Taneyama M., *Okamoto T., Yamane H., Minamikawa T. (2001) Involvement of Gibberellins in Expression of a Cysteine Proteinase (SH-EP) in Cotyledons of Vigna mungo Seedlings. Plant Cell Physiol. 42: 1290-1293.
Toyooka K., *Okamoto T., Minamikawa T. (2001) Cotyledon cells of Vigna mungo seedlings utilize at least two distinct autophagic machineries for degradation of starch granules and cellular components. J. Cell Biol. 154: 973-982.
Tsuru-Furuno A., *Okamoto T., Minamikawa T. (2001) Isolation of a putative receptor for KDEL-tailed cysteine proteinase (SH-EP) from cotyledons of Vigna mungo seedlings. Plant Cell Physiol. 42: 1062-1070.
Toyooka K., *Okamoto T., Minamikawa T. (2000) Mass transport of a KDEL-tailed cysteine proteinase (SH-EP) to protein storage vacuole by ER-derived vesicle is involved in protein mobilization in germinating seeds. J. Cell Biol. 148: 453-463.
*Okamoto, T., T. Minamikawa, C. Edward, V. Vakharia and E. Herman (1999) Posttranlational removal of the carboxyterminal KDEL of the cysteine protease SH-EP occurs prior to maturation of the enzyme. J. Biol. Chem. 274: 11390-11398.
*Okamoto T., Yuki A., Mitsuhashi N. and Minamikawa T. (1999) Asparaginyl endopeptidase (VmPE-1) and autocatalytic processing synergistically activate the vacuolar cysteine proteinase (SH-EP). Eur. J. Biochem. 264: 223-32.
*Okamoto T., Minamikawa T. (1999) Molecular cloning and characterization of Vigna mungo processing enzyme 1 (VmPE-1), an asparaginyl endopeptidase possibly involved in post-translational processing of a vacuolar cysteine endopeptidase (SH-EP). Plant Mol. Biol. 39: 63-73.
Hosokawa T., *Okamoto T., Minamikawa T. (1999) Characterization of serine endopeptidases in cotyledons of germinated Vigna mungo seeds. J. Plant Res. 112: 217-221.
Zhong P-Y., *Yamauchi D., Okamoto T., Okabe T. and Minamikawa T. (1999) Synthesis and degradation of a 28-kDa pod storage protein in French bean (Phaseolus vulgaris) plants. Planta 210: 72-79.
*Okamoto T., Miura-Izu Y., Ishii S., Minamikawa T. (1996) Asparaginyl endopeptidase in developing and germinating legume seeds: Immunological detection and quantitation. Plant Sci. 115: 49-57.
Taneyama T., *Okamoto T., Yamauchi D., Minamikawa T. (1996) Development of endo-peptidase activity in cotyledons of Vigna mungo seedlings: Effects of exogenously applied end-products and plant hormones. Plant Cell Physiol. 37: 19-26.
*Yamauchi D., Terasaki Y., Okamoto T., Minamikawa T. (1996) Promoter r
egions of cysteine endopeptidase genes from legumes confer germination-specific expression in transgenic tobacco seeds. Plant Mol. Biol. 30: 321-329.
*Okamoto T., Minamikawa T. (1995) Purification of a processing enzyme (VmPE-1) that is involved in post-translational processing of a plant cysteine endopeptidase (SH-EP). Eur. J. Biochem. 231: 300-305.
Okamoto T., Nakayama H., Seta K., Isobe T., *Minamikawa T. (1994) Posttranslational processing of a carboxy-terminal propeptide containing a KDEL sequence of plant vacuolar cysteine endopeptidase (SH-EP). FEBS Lett. 351: 31-34.
総説・その他
*Toda E., *Okamoto T. (2020) Polyspermy in angiosperms: Its contribution to polyploid formation and speciation. Mol Reprod Dev. 87: 374-379.
*Okamoto T., Ohnishi Y., Toda E. (2017) Development of polyspermic zygote and possible contribution of polyspermy to polyploid formation in angiosperms. J. Plant Res., 130: 485-490. doi: 10.1007/s10265-017-0913-9
*Okamoto, T. (2014) Gene and protein expression profiles in rice gametes and zygotes: a cue for understanding the mechanisms in gametic and/or early zygotic development of angiosperms. In “Sexual Reproduction in Animals and Plants”, Eds, Sawada H., Inoue H., Iwano M., Springer, pp369-382.
*Okamoto T. (2006) Transport of proteases to the vacuole: ER export by-passing golgi? D.G. Robinson ed. The Plant Endoplasmic Reticulum. Plant Cell Monogr. 4: 125-139.
Okamoto T., *Kranz E. (2005) In vitro fertilization - a tool to dissect cell specification from a higher plant zygote. Embryology of Seed Plants: Then and Now. Professor Panchanan Maheshwari Centenary Special Issue of Current Science. 89: 1861-1869.
Okamoto T., *Kranz E. (2005) Major proteins in plant and animal eggs. Acta Biologica Cracoviensia, Series Botanica, 47, 17-22.
*Kranz E., Hoshino Y., Okamoto T., Scholten S. (2004) Double fertilization in vitro and transgene technology. Plant Biotechnology and Molecular Markers. Srivastava, P.S.; Narula, Alka and Srivastava, Sheela (eds), Kluwer Academic Publishes, Dordrecht, pp. 31-42.
*Okamoto T., Minamikawa T. (1998) A vacuolar cysteine endopeptidase (SH-EP) that digests seed storage globulin: characterization, regulation of gene expression, and posttranslational processing. J. Plant Physiol. 152: 675-682.
*Okamoto T., Minamikawa T. (1997) A cysteine endopeptidase (SH-EP) in geminatedVigna mungo seeds; post-translational processing and intracellular transport. Basic and Applied Aspects of Seed Biology. Kluwer Academic Publishers. pp563-568.
加藤 紀夫、岡本 龍史 (2021) 「植物受精卵でのゲノム編集技術の開発と品種改良への応用とその課題」、ゲノム編集技術を応用した製品開発とその実用化(技術情報協会)、第7章2節 pp522-528
岡本 龍史、戸田絵梨香、加藤 紀夫 (2020) 「植物受精卵を用いたゲノム編集」、植物の化学調節、55 (1) 52-56.
加藤 紀夫、戸田絵梨香、岡本 龍史 (2020) 「イネ受精卵への一過的導入法」、進化するゲノム編集vol. II(NTS出版)、第3章 pp27-31
戸田絵梨香、岡本 龍史 (2019) 「イネ受精卵発生過程における雌雄配偶子の機能差および父性アリル依存的遺伝子発現による受精卵の発生誘導」、アグリバイオ2019年4月号、pp 73-78.
岡本龍史 (2017) in vitro 受精系と育種、「受精後雑種障壁研究の育種利用に向けて」、育種学研究、 Vol. 19 (No. 1) p. 35-36.
井川智子、東山哲也、岡本龍史 (2014)「第7章:被子植物の受精2:花粉管の伸長とガイダンス、配偶子の融合、核の合一」、動植物の受精学:共通機構と多様性、澤田均編、化学同人、pp103-118.
岡本龍史 (2009) 受精の生理、種子の科学とバイオテクノロジー、原田久也他/編、学会出版センター、pp25-27.
岡本龍史 (2006) 胚発生、植物ホルモンの分子細胞生物学、小柴共一・神谷勇治/編、講談社、pp122-134.
岡本龍史、内海貴夫(2006) In vitro 受精系を用いた胚発生研究法、植物の生長調節 41:75-86.
岡本龍史 (2002) KDEL小胞を介した液胞タンパク質の輸送系.植物細胞工学シリーズ17: 42-50.
岡本龍史、南川隆雄 (2002) 発芽種子の貯蔵物質分解機構:液胞加水分解酵素・KDEL小胞・オートファジー.化学と生物40: 790-798.