|
|
 Developmental Biology Laboratory
Developmental Biology Laboratory
|
Our laboratory is dedicated to identifying the molecular/genetic basis of the developmental program for chordate embryos and understanding how the developmental program is executed during the course of development from a single cell, fertilized egg to the adult form with functional tissues and organs.
|
|
Asc Prof |
Kimiko Fukuda |
e-mail |
|
|
Asc Prof |
Naohito Takatori |
e-mail |
|
*Visiting Professor
|
|
The mechanism involved in differentiation of the digestive tract
|
|

Expression of liver marker gene, Hex in the foregut
The digestive tract, which connects between the mouth and the anus, differentiates into various digestive organs. We are interested in the molecular mechanism underlying following topics during development of the digestive tract.
1, Differentiation of the endoderm, which will give rise to gut epithelium.
2, Epithelial folding at the onset of the gut tube formation.
3, Endodermal regionalization, prior to organ differentiation.
4, Boundary formation between two digestive organs.
5, Variation of the digestive organs among species.
|
|
|
Study on the mechanism that segregates mesoderm and endoderm fates in chordate embryos.
|
|
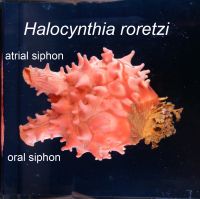
The various types of cells that constitute our body is created from a single cell, the fertilized egg. Establishment of the three germ layers, ectoderm, mesoderm and endoderm, is an important early step that is required for the creation of various types of cells. The mechanism that establishes the germ layers have been deeply studied ever since the early days of developmental biology. Recent analysis have shown that mesoderm and endoderm arise from a common ancestor, the mesendoderm cells. However, it is still not understood well how the mesoderm and endoderm fates are separated into different mesendoderm decedent cells.
We are tackling this problem using a marine animal called ascidians (Halocynthia roretzi). Ascidians are sessile filter feeders that spend most of their life attached to objects at the sea bottom, such as rocks and ropes. The opening at the lower left of the animal shown in the photograph is the oral siphon where sea water enters the body. Planktons and organic materials are filtered within the body and the left over sea water leaves through the opening in the upper left, the atrial siphon. The atrial siphon is also used for spawning. Ascidians, surprising for their appearance, is our close relative: ascidians belong to the chordate phylum which includes human, chicks, frogs and fish, but not insects, molluscs or sea urchin. Our favorite animal is not famous, but has many characteristics which have helped us in conducting research.
Our studies have shown that mesoderm and endoderm fates are separated by the following sequence of events. First, mesendoderm cell nucleus moves to the future mesoderm forming side of the cell. mRNA encoding a transcription factor, Not, is transcribed and stored in the nucleus during the migration. The cell enters M-phase when the nucleus is positioned near the mesoderm pole, and Not mRNA is released to the mesoderm side cytoplasm. The mitotic spindle returns to the center of the cell, but Not mRNA remains in the mesoderm side. Not mRNA is partitioned to the mesoderm daughter cell, where it is translated and Not transcription factor suppresses endoderm fate and promotes mesoderm fate. We also found that the direction of nuclear migration is determined by the localization of PI3K and its product, PIP3, to the mesoderm side. We are currently investigating the mechanism that localizes PI3K and PIP3 to the mesoderm side.
|
|
|
|
|
Recent Publications
|
|
- Asai, R., Haneda, Y., Seya, D., Arima, Y., Fukuda, K., Kurihara, Y., Miyagawa-Tomita, S., and Kurihara, H. (2017) Amniogenic somatopleure: a novel origin of multiple cell lineages contributing to the cardiovascular system. Scientific Reports 7, 8955
- Takatori, N., Oonuma, K., Nishida, H., and Saiga, H. (2015). Polarization of PI3K Activity Initiated by Ooplasmic Segregation Guides Nuclear Migration in the Mesendoderm. Dev Cell 35, 333-343.
- Oonuma, K., Hirose, D., Takatori, N. and Saiga, H. (2014) Analysis of the transcription regulatory mechanism of Otx during the development of the sensory vesicle in Ciona intestinalis. Zool. Sci. 31, 565-572.
- Oonuma, K., Hirose, D., Takatori, N. and Saiga, H. (2014) Continuous expression of Otx in the anterior neural lineage is supported by different transcriptional regulatory mechanisms during the development of Halocynthia roretzi. Dev. Growth & Differ. 56, 189-198.
- Kimura, W., Cantas, A. Sheng, G. Jakt, M., Yasugi, S. and Fukuda, K. (2011) Identification of region specific genes in early chicken endoderm. Gene Exp. Patterns (in press).
- Kumano, G., Takatori, N., Negishi, T., Takada, T., and Nishida, H. (2011). A maternal factor unique to ascidians silences the germline via binding to P-TEFb and RNAP II regulation. Curr Biol 21, 1308-1313.
- Takatori, N., Kumano, G., Saiga, H. and Nishida, H. (2010) Segregation of germ layer fates by nuclear migration-dependent localization of Not mRNA. Dev. Cell 19, 589-598.
- Kobayashi, M., Takatori, N., Nakajima, Y., Kumano, G., Nishida, H. and Saiga, H. (2010) Spatial and temporal expression of two transcriptional isoforms of Lhx3, a LIM class homeobox gene, during embryogenesis of two phylogenetically remote ascidians, Halocynthia roretzi and Ciona intestinalis. Gene Exp. Patterns, 10, 98-104.
- Niwano, T., Takatori, N., Kumano, G., and Nishida, H. (2009). Wnt5 is required for notochord cell intercalation in the ascidian Halocynthia roretzi. Biol Cell 101, 645-659.
- Takatori, N., Butts, T., Candiani, S., Pestarino, M., Ferrier, D.E.K., Saiga, H. and Holland, P.W.H. (2008) Comprehensive survey and classification of homeobox genes in the genome of amphioxus, Branchiostoma floridae. Dev. Genes Evol. 218, 570-590.
- Holland, L.Z., Albalat, R., Azumi,K., Benito-Gutiérrez, E., Blow, M.J., Bronner-Fraser, M., Brunet, F., Butts, T., Candiani, S., Dishaw, L.J., Ferrier, D.E.K., Garcia-Fernàndez, J., Gibson-Brown, J.J., Gissi, C., Godzik, A., Hallböök, F., Hirose, D., Hosomichi, K., Ikuta, T., Inoko, H., Kasahara, M., Kasamatsu, J., Kawashima, T., Kimura, A., Kobayashi, M., Kozmik, Z., Kubokawa,K., Laudet,V., Litman, G.W., McHardy, A.C., Meulemans, D., Nonaka, M., Olinski, R.P., Pancer, Z., Pennacchio, L.A., Pestarino, M., Rast, J.P., Rigoutsos, I., Robinson-Rechavi, M., Roch, G., Saiga, H., Sasakura, Y., Satake, M., Satou, Y., Schubert, M., Sherwood, N., Shiina, T., Takatori, N,, Tello, J., Vopalensky, P., Wada, S., Xu, A., Ye, Y., Yoshida, K., Yoshizaki, F., Jr-Kai, Y., Zhang, Q., Zmasek, C.M., Putnam, N.H., Rokhsar, D.S., Satoh, N. and Holland, P.W.H.(2008) The amphioxus genome illuminates vertebrate origins and cephalochordate biology. Genome Res. 18, 1100-1111.
- Takatori, N. and Saiga, H. (2008) Evolution of CUT class homeobox genes: insights from the genome of the amphioxus, Branchiostoma floridae. Int. J. Dev. Biol. 52, 969-977.
- Takatori, N., Wada, S. and Saiga, H. (2007) Regionalization of the tail-tip epidermis requires inductive influence from vegetal cells and FGF signaling in the development of an ascidian, Halocynthia roretzi. Zool. Sci. 24, 441-448.
- Kimura, W., Yasugi, S. and Fukuda, K. (2007) Regional specification of the endoderm in the early chick embryo. Dev. Growth & Differ. 49, 365-372.
- Hojo, M., Takada, I., Kimura, W., Fukuda, K. and Yasugi, S. (2006) Expression patterns of the chicken peroxisome proliferator-activated receptors (PPARs) during the development of the digestive organs. Gene Expression Patterns 6, 171-179.
- Shin, M., Noji, S., NeubuNsser, A. and Yasugi, S. (2006) FGF10 is required for cell proliferation and gland formation in the stomach epithelium of the chicken embryo. Dev. Biol. 294, 11-23.
- Kimura, W., Yasugi, S., Stern, C. and Fukuda, K. (2006) Fate and plasticity of the endoderm in the early chick embryo. Dev. Biol. 289, 283-295.
- Matsuda, Y., Wakamatsu, Y., Kohyama, J., Okano, H., Fikuda, K. and Yasugi, S. (2005) Notch signaling functions as a binary switch for the determination of glandular and luminal fates of endodermal epithelium during chicken stomach development. Development 132, 2783-2793.
- Yagi, K., Takatori, N., Satou, Y., and Satoh, N. (2005). Ci-Tbx6b and Ci-Tbx6c are key mediators of the maternal effect gene Ci-macho1 in muscle cell differentiation in Ciona intestinalis embryos. Dev Biol 282, 535-549.
- Asai, R., Okano, H. and Yasugi, S. (2005) Correlation between Musashi-1 and c-hairy-1 expression and cell proliferation activity in the developing intestine and stomach of both chicken and mouse. Dev. Growth & Differ. 47, 501-510.
- Hoshino, A., Koide, M., Ono, t. and Yasugi, S. (2005) Sex-specific and left-right asymmetric expression pattern of Bmp7 in the gonad of normal and sex-reversed chicken embryos. Dev. Growth Differ. 47, 65-74.
- Hojo, M., Takada, I., Kimura, W., Fukuda, K. and Yasugi, S. (2005) Expression patterns of the chicken peroxisome proliferator-activated receptors (PPARs) during the development of the digestive organs. Gene Expression Patterns 6, 171-179.
- Shin, M., Watanuki, K. and Yasugi, S. (2005) Expression of Fgf10 and Fgf receptors during development of the embryonic chicken stomach. Gene Expression Patterns 5, 511-516.
- Hiramatsu, H. and Yasugi, S. (2004) Molecular analysis of the determination of developmental fate in the small Intestinal epithelium in the chicken embryo. Int. J. Dev. Biol. 48: 1141-1148.
- Takatori, N., Hotta, K., Mochizuki, Y., Satoh, G., Mitani, Y., Satoh, N., Satou, Y., and Takahashi, H. (2004). T-box genes in the ascidian Ciona intestinalis: characterization of cDNAs and spatial expression. Dev Dyn 230, 743-753.
- Shimizu, Y., Yamamichi, N., Saitoh, K., Watanabe, A., Ito, M., Yamamichi-Nishina, M., Mizutani, M., Yahagi, N., Suzuki, T., Sasakawa, C., Yasugi, S., Ichinose, M. and Iba, H. (2003). Kinetics of v-src-induced epithelial-mesenchymal transition in developing glandular stomach. Oncogene 22, 884-893.
- Saito, K., Kawaguchi, A., Kashiwagi, S., Yasugi, S., Ogawa, M. and Miyata, T. (2003) Morphological asymmetry in dividing retinal progenitor cells. Dev. Growth & Differ. 45: 219-229.
- Fukuda, K., Kameda, T., Saitoh, K., Iba, H. and Yasugi, S. (2003) Down-regulation of endodermal Shh is required for gland formation in chicken stomach. Mech. Dev. 120: 801-809.
- Shin, M., Fukuda, K. and Yasugi, S. (2003) Expression of DDSG1, a novel gene encoding a putative DNA-binding protein in the embryonic chicken nervous system. Mech. Dev. Gene Expression 3, 431-436.
- Watanuki, K. and Yasugi, S. (2003) Analysis of transcription regulatory regions of embryonic chicken pepsinogen (ECPg) gene. Dev. Dyn. 228: 51-58.
- Sasakura, Y., Yamada, L., Takatori, N., Satou, Y., and Satoh, N. (2003). A genomewide survey of developmentally relevant genes in Ciona intestinalis. VII. Molecules involved in the regulation of cell polarity and actin dynamics. Dev Genes Evol 213, 273-283.
- Sasakura, Y., Shoguchi, E., Takatori, N., Wada, S., Meinertzhagen, I.A., Satou, Y., and Satoh, N. (2003). A genomewide survey of developmentally relevant genes in Ciona intestinalis. X. Genes for cell junctions and extracellular matrix. Dev Genes Evol 213, 303-313.
- Dehal, P., Satou, Y., Campbell, R.K., Chapman, J., Degnan, B., De Tomaso, A., Davidson, B., Di Gregorio, A., Gelpke, M., Goodstein, D., Harafuji, N., Hastings, K., Ho, I., Hotta, K., Huang, W., Kawashima, T., Lemaire, P., Martinez, D., Meinertzhagen, I. A., Necula, S., Nonaka, M., Putnam, N., Rash, S., Saiga, H., Satake, M., Terry, A., Yamada, L., Wang H.-G., Awazu A., Azumi K., Boore J., Branno M., Chin-bow S., DeSantis R., Doyle, S., Francino, P., Keys, D., Haga, S., Hayashi, H., Hino, K., Imai, K. S., Inaba, K., Kano, S., Kobayashi, K., Kobayashi, M., Lee, B.-I., Makabe, K.W., Manohar, C., Matassi, G., Medina, M., Mochizuki, Y., Mount, S., Morishita, T., Miura, S., Nakayama, A., Nishizaka, S., Nomoto, H., Ohta, F., Oishi, K., Rigoutsos, I., Sano, M., Sasaki, A., Sasakura, Y., Shoguchi, E., Sin-I, T., Spagnuolo, A., Stainier, D., Suzuki, M.M., Tassy, O., Takatori. N., Tokuoka, M., Yagi, K., Yoshizaki, F., Wada, S., Zhang, C., Hyatt, P.D., Larimer, F., Detter, C., Doggett, N., Glavina, T.,Hawkins, T.,Richardson, P., Lucas, S.,Kohara, Y., Levine, M., Satoh N. and Rokhsar, D. (2002) The draft genome of Ciona intestinalis: insights into chordate and vertebrate origins. Science 298, 2157-2167.
- Takatori, N., Satou, Y., and Satoh, N. (2002). Expression of hedgehog genes in Ciona intestinalis embryos. Mech Dev 116, 235-238.
- Satou, Y., Yamada, L., Mochizuki, Y., Takatori, N., Kawashima, T., Sasaki, A., Hamaguchi, M., Awazu, S., Yagi, K., Sasakura, Y., et al. (2002). A cDNA resource from the basal chordate Ciona intestinalis. Genesis 33, 153-154.
- Satou, Y., Takatori, N., Fujiwara, S., Nishikata, T., Saiga, H., Kusakabe, T. Shin-I, T., Kohara, Y. and Satoh, N.(2002) Ciona intestinalis cDNA projects: expressed sequence tag analyses and gene expression profiles during embryogenesis. Gene 287, 83-96.
- Satou, Y., and Satoh, N. (2002). Gene expression profiles in Ciona intestinalis cleavage-stage embryos. Mech Dev 112, 115-127.
- Fukuda, K. and Yasugi, S. (2002) Versatile roles for sonic hedgehog in gut development. J. Gastroenterol. 37, 239-246.
- Matsushita, S., Ishii, Y., Scotting, P. J. and Yasugi, S. (2002). The pre-gut endoderm of chick embryos is regionalized by 1.5 days of development. Dev. Dyn. 223, 33-47.
- Yasugi, E., Uemura, I., Kumagai, T., Nishikawa, Y., Yasugi, S.. and Yuo, A. (2002). Disruption of mitochondria is an early event during dolichyl monophosphate-induced apoptosis in U937 cells.@Zool. Sci. 19: 7-13.
- Takeda, J., Tabata, H., Fukuda, K. and Yasugi, S. (2002). The involvement of signal transduction pathway mediated by epidermal growth factor receptor in the differentiation of chicken glandular stomach. Dev. Growth & Differ. 44: 501-508
- Satou, Y., Takatori, N., Yamada, L., Mochizuki, Y., Hamaguchi, M., Ishikawa, H., Chiba, S., Imai, K., Kano, S., Murakami, S.D., et al. (2001). Gene expression profiles in Ciona intestinalis tailbud embryos. Development 128, 2893-2904.
- Hotta, K., Takahashi, H., Asakura, T., Saitoh, B., Takatori, N., Satou, Y., and Satoh, N. (2000). Characterization of Brachyury-downstream notochord genes in the Ciona intestinalis embryo. Dev Biol 224, 69-80.
|
|
|